- Visibility 14 Views
- Downloads 5 Downloads
- DOI 10.18231/j.ijooo.2021.016
-
CrossMark
- Citation
Clinical study: Evaluation of the effect of topical nepafenac on central foveal thickness and visual acuity post panretinal photocoagulation in diabetic retinopathy
- Author Details:
-
idyashree VA
-
Ravi B *
-
Shashidhar Swamy
-
Vishwanath B N
-
Swetha Kumari
Introduction/Background
Diabetes Melletus is a metabolic disorder characterised by chronic hyperglycemia associated with disturbances in metabolism of carbohydrates, proteins and fats as a result of defect in the secretion of insulin or its action.[1]
India is known as the “diabetic capital of the world”. It is estimated that, by 2030 the diabetic population in India will rise to 79.4 million. Diabetic retinopathy is becoming an important cause of blindness in India. It was 17th cause of blindness 20 years back and now it ascended to 6th position.
Diabetic retinopathy is one of the microvascular complications of DM which affects 1 out of 3 patients with diabetes resulting in poor quality of life in patients with severe DR.[2] Diabetic retinopathy is the leading cause of blindness among the age group of 25-40 years in the industrialised world. Chronic hyperglycemia is the primary factor responsible for the development of diabetic retinopathy and the other complications of diabetes. The importance of long term glycemic control and the decrease in the complications of diabetes is conclusively established by the landmark clinical trials like the Diabetes Control and Complications Trial (DCCT) and the UK prospective Diabetes Study (UKPDS).[3], [4]
Proliferative diabetic retinopathy (PDR) is characterized by neovascularization, including neovascularization on disc, neovascularisation elsewhere which leads to vitreous hemorrhage and neovascularisation of iris which may lead to neovascular glaucoma.[5]
Laser photocoagulation is the most common treatment method employed for proliferative diabetic retinopathy. The laser therapy in the form of panretinal photocoagulation where about 2000 large burns each measuring about 500µm of greater intensity are applied on to retina outside the central thirty-degree zones. This tends to decrease the caliber of new vessels and helps in regression of new vessels.[6]
Macular edema is main side-effect of panretinal photocoagulation and may be sufficiently severe to reduce the visual acuity.[6] Inflammation and altered blood flow are the possible mechanisms responsible for the development of post panretinal photocoagulation macular edema.[7]
Topical treatment with nepafenac (NSAID) is known to produce significant decline in diabetes induced biochemical alterations, including retinal PGE2, CYCLO-OXYGENASE 2, superoxide production.[8] In addition, cellular and morphological changes such as leucostasis, retinal capillary degeneration, endothelial cell apoptosis were inhibited by Nepafenac.[9] Our study aims at studying the effect of topical Nepafenac in preventing the development of macular edema following PRP.
Materials and Methods
Our study was a hospital based randomized control study, conducted from November 2018 to May 2020, at our tertiary eye hospital. A total of 68 eyes of 66 patients with proliferative diabetic retinopathy without pre-existing macular edema were included in the study, after taking informed consent. The study was planned and conducted in accordance with the Helsinki Declaration. Institution ethical committee approval was taken. All patients of PDR without macular edema and those requiring PRP were included in the study. Patients with dry eye syndromes, corneal epithelial defect, with prior macular edema, advanced diabetic eye disease, ocular media opacity, prior history of ocular surgery or laser treatment and patients allergic to NSAIDS were excluded from study. Detailed ocular examination included Recording of Visual acuity using Snellen’s chart/ LogMAR chart, Examination of the adnexa and extraocular structures- Face, orbits, eyebrows, eyelids, palpebral fissure, extra ocular movements, lacrimal sac, Examination of Anterior segment by slit lamp biomicroscopy, Examination of Posterior segment by ophthalmoscopy (direct and indirect) and +90 D Biomicroscopy. Central foveal thickenss was measured using Spectral domain OCT (Cirrus 5000, Carl Zeiss Meditec, Inc, Dublin, USA) pre and post panretinal photocoagulation of all the patients. Fundus photography and fundus fluroscein angiography were peorformed as and when necessary.
All the patients were divided into two equal groups using random tables
Pateints who were given nepafenac eye drops (test
Patients who were given carboxymethyl cellulose or moxiflox eye drops(placebo (control
All the patients in two groups were matched based on: age, sex, type of diabetes, HBA1C levels and duration of type II diabetes (defined as the duration from time of diagnosis upto the time of enrolment into the study).
PRPwas done to all the patients in 3 sessions within 3 weeks, with the parameters- retinal spot size 500µm, intensity 150-250mw until a grey white spot seen, duration of 0.1s, with 1000spots/eye in the first session, followed by 300-500 spots/eye in the subsequent two sessions. Following which, patients were followed-up at 1st month, 2nd month and 4th month. Efficacy parameters- best corrected visual acuity and central foveal thickness using HD OCT was measured at each visit.
Statisitcal analysis
The data is entered in MS excel and analysed using SPSS Ver 20.0. Descriptive statistics is analysed and represented using mean, median, standard deviation, standard error of mean and categorical variables are represented using percentages. Pearson Chi Square test and Fischer’s Exact test is used to measure association between two categorical variables, Independent sample test is used to measure statistically significant difference among two groups.
Observation and Results
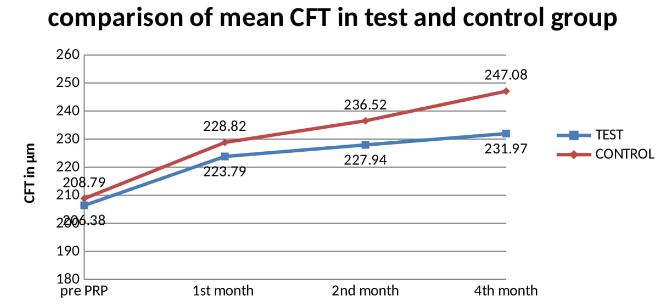
Study was conducted on 66 eyes of 68 patients. There were 19 males (55.88%) in test group and 20 males (58.82%) in control group. ([Table 1]) Mean age was 62.20 years in test group and 61.79 years in control group. Mean HBA1c in test group was 8.57% and in control group was 8.78% which is statistically insignificant between the groups. 19 patients in test group and 15 patients in control group had systemic hypertension. Mean duration of Diabetes was 15.58years in test group and 15.29years in control group which is comparable. ([Table 1]) Mean foveal thickness in test group at baseline (Pre PRP), month 1, month 2 and month 4 after PRP was 206.38±6.38 microns, 223.79±9.31microns, 227.94±9.70 microns and 231.97±10.06 microns respectively. Mean foveal thickness in control group at baseline (Pre PRP), month 1, month 2 and month 4 after PRP was 208.79±7.31 microns, 228.82±10.23 microns, 236.52±12.12 microns and 247.08±15.78 microns respectively. There was a significant difference in CFT between test and control group. ([Table 2]) Mean best corrected visual acuity in test group at baseline (Pre PRP), month 1, month 2 and month 4 after PRP was 0.97.38±0.11 logMAR, 1.04±0.14 logMAR, 1.04±0.14 logMAR and 1.05±0.14 logMAR respectively. Mean best corrected visual acuity in control group at baseline (Pre PRP), month 1, month 2 and month 4 after PRP was 0.96.38±0.14 logMAR, 1.08±0.16 logMAR, 1.09±0.16 logMAR and 1.13±0.19 logMAR respectively. There was no statistically difference in BCVA between the groups at any of the follow up. ([Table 3]) Four eyes (11.76%) in each group reported blurred vision following PRP. three patients (8.82%) in test group and one patient (2.94%) in control group reported itching following PRP. One patient (2.94%) in each group reported eye pain following PRP. ([Table 4])
|
Variable |
Test |
Control |
P value |
||
|
Gender distribution |
Males |
Females |
Males |
Females |
|
|
19 |
15 |
20 |
14 |
||
|
Mean age |
62.20 years |
61.79 years |
0.27 |
||
|
Mean hba1c |
8.57% |
8.78% |
0.507 |
||
|
Hypertension |
19 patients |
15 patients |
0.332 |
||
|
Mean duration of diabetes |
15.58 years |
15.29 years |
0.714 |
|
|
Pre PRP |
1st month |
2nd month |
4th month |
|
Test(mean±SD) |
206.38 ± 6.38 |
223.79 ± 9.31 |
227.94 ± 9.70 |
231.97 ± 10.06 |
|
Control (mean ±SD) |
208.79 ± 7.31 |
228.82 ± 10.23 |
236.52 ± 12.12 |
247.08 ± 15.78 |
|
P Value |
0.152 |
0.042 |
0.002 |
0.0001 |
|
|
Pre PRP |
1 st month |
2 nd month |
4 th month |
|
Test (mean±SD) |
0.97 ± 0.11 |
1.04 ± 0.14 |
1.04 ± 0.14 |
1.05 ± 0.14 |
|
Control (mean ±SD) |
0.96 ± 0.14 |
1.08 ± 0.16 |
1.09 ± 0.16 |
1.13 ± 0.19 |
|
P Value |
0.840 |
0.211 |
0.204 |
0.068 |
|
Adverse events |
Test |
Control |
|
Blurred vision |
4(11.76%) |
4(11.76%) |
|
Itching |
3(8.82%) |
1(2.94%) |
|
Eye pain |
1(2.94%) |
1(2.94%) |
|
Eye discharge |
1(2.94%) |
2(5.88%) |
|
Conjunctivitis |
0(0%) |
1(2.94%) |
|
Punctate keratitis |
1(2.94%) |
0(0%) |
|
Chronic redness |
3(8.82%) |
3(8.82%) |
|
Mean Central foveal thickness |
Pre PRP |
1st month |
2nd month |
4th month |
||||
|
Test |
Control |
Test |
Control |
Test |
Control |
Test |
Control |
|
|
Our study |
206.38 |
208.79 |
223.79 |
228.82 |
227.94 |
236.52 |
231.90 |
247.08 |
|
Nahla B. Abu Hussein |
191.63 |
182.68 |
200.44 |
204.18 |
205.56 |
210.25 |
208.22 |
219.68 |
|
BCVA |
Pre PRP |
1st month |
2nd month |
4th month |
||||
|
|
Test |
Control |
Test |
Control |
Test |
Control |
Test |
Control |
|
Our study |
0.97 |
0.96 |
1.04 |
1.08 |
1.04 |
1.09 |
1.05 |
1.13 |
|
Nahla B. Abu Hussein |
0.06 |
0.07 |
0.09 |
0.14 |
0.09 |
0.17 |
0.11 |
0.19 |
|
|
Blurred Vision |
Itching |
Eye pain |
Discharge |
Conjunctivitis |
Punctate keratitis |
Chronic redness |
|||||||
|
T |
C |
T |
C |
T |
C |
T |
C |
T |
C |
T |
C |
T |
C |
|
|
Our study |
4 |
4 |
3 |
1 |
1 |
1 |
1 |
2 |
0 |
1 |
1 |
0 |
3 |
3 |
|
Nahla B Abu Hussain et al |
5 |
3 |
2 |
1 |
1 |
1 |
1 |
2 |
0 |
1 |
0 |
0 |
3 |
3 |
Discussion
Diabetes mellitus is one the important etiological factor in causation of preventable blindness. As there is increasing in the prevalence of diabetes, burden of blindness in going to increase. There are various causes for reduction of visual acuity which includes Cataract, Macular edema, tractional retinal detachment, vireous hemorrhage and neovascular glaucoma. PRP is one of the key treatment tool for management of proliferative diabetic retinopathy in developing countries like India. However there are certain complications which occurs with PRP.
In a study conducted by Watanachai N et al [10] it was observed that following a single session multispot PRP, there was increase in macular thickness after 1 week of PRP with corresponding mild change in BCVA. Similarly in the study conducted by Mukhthar A [11] it was concluded that there is change in central macular thickness and BCVA as measured by OCT following 2 sessions of PRP with PASCAL at the interval of 4 weeks in between the 2 sessions.
Macular edema is main side-effect of panretinal photocoagulation and may be sufficiently severe to reduce the visual acuity.[6] Inflammation and altered vascular permeability are the possible mechanisms responsible for the development of post panretinal photocoagulation macular edema.[7]
Topical treatment with Nepafenac (NSAID) is known to produce significant decline in diabetes induced biochemical alterations, including retinal PGE2, CYCLO-OXYGENASE 2 and superoxide production[12] In addition, cellular and morphological changes such as leucostasis, retinal capillary degeneration, endothelial cell apoptosis were inhibited by Nepafenac.[8]
A study conducted by David Callanan et al [9] showed decrease in macular edema as measured by OCT with the use of topical nepafenac twice daily. In our study we have used topical nepafenac to decrease the macular thickness following PRP. The results obtained from our study is similar to the results of the study conducted by Abu Hussein et al.[12]
68 eyes of 66 patients were included in our study, in which 34 eyes were divided into test and control group each. Every patient underwent PRP in 3 sessions at the interval of 1 week, and were followed-up at 1 month, 2 months and 4 months. CFT was more in control group as compared to test group and this change was statisitically significant. Change in BCVA was significant clinically but not statisitically between the groups during follow up.
The changes in central foveal thickness noted during the follow-up in our study and the study conducted by Nahla B. Abu Husseinare are shown in table 6. Similarly the BCVA in our study showed clinically significant changes but was statistically insignificant.
The difference in the BCVA in our study and the study conducted by Nahla B. Abu Husseincan be explained by the observation that the mean age of presentation in our study was 62 years, most of the patients were with the advanced diabetic eye disease and few patients developed cataract during the follow-up. Late presentation due to the lack of proper awareness on the routine screening programmes for diabetic retinopathy was also observed during the study. Non-compliance with medication was also noted in few patients during the follow-up. Comparison of ocular adverse events between our study and the study conducted by Nahla B. Abu Hussein did not show any significant difference.([Table 6])
Conclusion
Using topical nepafenac eye drops following PRP for proliferative diabetic retinopathy is effective in reducing central foveal thickness and preventing the corresponding worsening of BCVA.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Source of Funding
None.
References
- . Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision: results of a randomized trial-Diabetic Retinopathy Vitrectomy Study report 3. Ophthalmol 1988. [Google Scholar]
- . Task Force on Diabetic Eye Care Committee. ICO Guidelines for Diabetic Eye Care. 2014. [Google Scholar]
- . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993. [Google Scholar]
- . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998. [Google Scholar]
- K Gündüz, S J Bakri. Management of proliferative diabetic retinopathy. Compr Ophthalmol Update 2007. [Google Scholar]
- J G Dowler. Laser management of diabetic retinopathy. J Royal Soc Med 2003. [Google Scholar]
- RP Singh, G Staurenghi, A Pollack, A Adewale, T Walker, D Sager. Efficacy of nepafenac ophthalmic suspension 0.1% in improving clinical outcomes following cataract surgery in patients with diabetes: an analysis of two randomized studies. Clin Ophthalmol 2017. [Google Scholar] [Crossref]
- TS Kern, CM Miller, Y Du, L Zheng, S Mohr, SL Ball. Topical Administration of Nepafenac Inhibits Diabetes-Induced Retinal Microvascular Disease and Underlying Abnormalities of Retinal Metabolism and Physiology. Diabetes 2007. [Google Scholar] [Crossref]
- D Callanan. Topical nepafenac in the treatment of diabetic macular edema. Clin Ophthalmol 2008. [Google Scholar] [Crossref]
- N Watanachai, J Choovuthayakorn, D Patikulsila, N Ittipunkul. Changes in Central Macular Thickness following Single Session Multispot Panretinal Photocoagulation. J Ophthalmol 2015. [Google Scholar] [Crossref]
- A Mukhtar, MS Khan, M Junejo, M Ishaq, B Akbar. Treatment of breast ductal carcinoma in situ (DCIS) as a rare entity in an unscreened population in a developing country. Pak J Med Sci 2016. [Google Scholar] [Crossref]
- N B Abu Hussein, AA Mohalhal, DA Ghalwash, AA Abdel-Kader. Effect of Topical Nepafenac on Central Foveal Thickness following Panretinal Photocoagulation in Diabetic Patients. J Ophthalmol 2017. [Google Scholar] [Crossref]
